Young Scientists of INRTU and A.E. Favorsky Irkutsk Institute of Chemistry SB RAS Have Developed a New Approach to Activation of Organic Molecules with the Help of Light and Published the Results in the Journal of the American Chemical Society
The Russian Science Day is celebrated on February 8. On this special day we present young scientists fr om the joint laboratory of INRTU and IrIC SB RAS, who have contributed to the development of new approaches to the activation of organic molecules using light and published their work in the high-ranking Journal of the American Chemical Society.
Organic chemistry lives by its own strict laws, which assign each molecule a set of properties and chemical reactions in which it can be involved. For example, every professional chemist generally knows what reactivity can be expected from a given compound. Therefore, inducing seemingly "impossible" chemical reactions is a very interesting area of research, leading to new ideas and discoveries.
Photochemical reactions are a suitable tool for temporarily "activating" molecules and forcing them to react in ways that were previously impossible. In recent years, leading teams of chemists from Germany and the USA have been working in this field, leading to the development of a new generation of materials with the ability to undergo controlled degradation and regeneration. Employees of the Laboratory of Photofunctional Materials (a joint unit of INRTU and IrIC SB RAS) contributed to this scientific direction and published their work in the highly reputable Journal of the American Chemical Society.
According to the head of the laboratory, Dr. Andrei Lvov, organic chemists know that it is not easy to create or break the bond between the oxygen atom and the benzene ring:

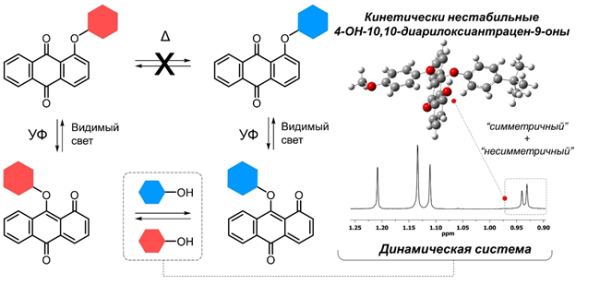
"Catalysis with transition metals, such as palladium salts in the Buchwald-Hartwig reaction, is necessary to create a C-O bond. Breaking this bond is particularly challenging, as in the case of diphenyl ether, wh ere two benzene rings are connected by an oxygen bridge. We have discovered a new approach to activate these structures and enable the exchange of phenoxy group fragments through the oxa Michael reaction of phenols with reactive ana-isomers of peri-aryloxyanthraquinones.
This discovery sheds light on a previously unknown aspect of the photochemistry of peri-aryloxyanthraquinones (PAHs), which was first observed by Soviet chemists in 1971 when they discovered the switching between para- and ana-isomers via arylotropy. Our research has demonstrated that the light-generated ana-isomers of PAH can interact with phenols through a previously unknown Michael oxa-reaction, resulting in the production of 4-hydroxo-10, 10-diaryloxyanthracene-9-ones (DAA). Calculations indicate that the reaction occurs through a cyclic transition state, with the simultaneous formation of C-O and O-H bonds and the breaking of another O-H bond. The DAA adducts are in thermal equilibrium with the PAH ana-isomers and disappear completely upon irradiation with visible light. This process enables the light-controlled substitution of one aryloxy group for another, which is impossible without activation of anthraquinone electrophilicity."
Vasily Bykov, a graduate student of INRTU, was the project's principal developer. He received assistance from Stepan Ukhanev, a graduate of the same university who is now a postgraduate student at IrIC SB RAS. Andrei Lvov points out that Professor Lubov Klimenko from Yugorsk State University (Khanty-Mansiysk) also contributed significantly to the work:
“She initiated research on photo-switchable peri-aryloxyanthraquinones in the USSR. Specifically, she studied the interaction of ana-isomers of anthraquinone with nucleophiles. Her expertise in this area greatly benefited this project. Additionally, her group synthesized the compound used in this work. Other co-authors of our work include Anna Vladimirovna Vologzhanina, Ph.D., from A.N.Nesmeyanov Institute of Organoelement Compounds of Russian Academy of Sciences (INEOS RAS), Moscow, who conducted the X-ray structural analysis, and Evgeny Antsiferov, Ph.D., who provided valuable assistance with cyclic voltammetry.”
The research was conducted under the state assignment of the Ministry of Education and Science of the Russian Federation, specifically by the Baikal research and education center and INEOS RAS.
Source: V.N. Bykov, S.A. Ukhanev, I.A. Ushakov, A.V. Vologzhanina, E.A. Antsiferov, L.S. Klimenko, and A.G. Lvov. The electrophilicity of Anthraquinone is activated by light, resulting in a dynamic C-O bond.
This was reported in the Journal of the American Chemical Society in 2024 (146:1799-1805).
The illustration provided by Andrei Lvov